Background
Intensive therapy with fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin (FLAG-IDA) is effective in young, fit AML patients with composite complete response (CRc) rates of 85% in de novo AML. FLAG-IDA is also commonly utilized in secondary AML (sAML) or relapsed/refractory (R/R) AML, where expected CRc rates are approximately 63% and 21%. Addition of the BCL-2 inhibitor venetoclax (VEN) to chemotherapy increases apoptosis priming, inferring improved responses may be possible when used in combination with FLAG-IDA.
Aim
The dual primary objectives were assessment of the safety and tolerability, and determination of dose limiting toxicities and the maximal tolerated dose of FLAG-IDA with VEN (FLAG-IDA-VEN). Secondary objectives included assessment of overall response rate (ORR: CR+CRi+CRh+MLFS+PR), CRc (CR+ CRh+CRi), OS, event free survival (EFS), duration of response (DOR), and biomarkers predictive of response or resistance to FLAG-IDA-VEN.
Methods
Patients age > 18 with treatment naïve/newly diagnosed (ND) or R/R AML were eligible. The phase Ib (P1b) dose escalation occurred as previously described in a cohort of patients with R/R AML (Abou Dalle ASH 2019). The phase II dose expansion included two arms: Arm A (P2A): ND AML, and Arm B (P2B): R/R AML, at the recommended phase 2 dosing.
Results
62 patients completed at least 1 cycle of therapy prior to analysis. Median age across cohorts was 51, 44, and 47 years. 18% of patients had sAML or therapy-related AML. ELN risk was favorable, intermediate, and adverse risk in 27%, 29%, and 44% of patients. R/R patients received a median of 2 (P1b; range 1-6) and 1 (P2B; range 1-2) prior lines of induction therapy. 50% of P1b and 32% of P2B patients underwent prior allogeneic stem cell transplantation (HSCT).
66% (N=41) of patients received 1-2 cycles of therapy; 34% (N=21) received ≥ 3 cycles. The most common reason for study discontinuation was transition to HSCT (N=31, 50%). Grade 3/4 adverse events occurring at a frequency ≥ 10% included febrile neutropenia (37%), bacteremia (29%), hypophosphatemia (24%), pneumonia (21%), hypokalemia (18%), skin/soft tissue infections (16%), and increased ALT (11%). 30 and 60-day mortality were 0 and 4.8%. Deaths on study have occurred only in R/R AML patients to date, secondary to sepsis (N=3), pneumonia (N=1), pulmonary hemorrhage (N=1), and the hemophagocytic syndrome (N=1).
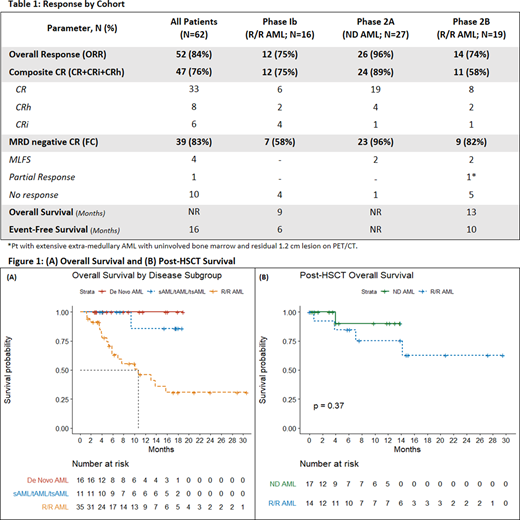
The ORR was 84%, with 89% (N= 24) of ND and 66% of R/R (N=23) patients achieving a CRc. 83% of patients (ND: 96%; R/R: 70%) achieved MRD negative CRc (MRD-) as assessed by flow cytometry. 100%, 85%, and 89% of ND and 83%, 60%, and 55% of R/R patients with ELN favorable, intermediate, and adverse risk disease achieved a CRc, respectively. 100% of patients with KMT2A rearrangements (N=7), and 50% of patients with extra-medullary AML (N=4) achieved a CRc. In R/R AML, mutations in tumor suppressor (TS) genes (TP53, WT1, PHF6) were more frequently identified in patients without response (66% vs. 19%, p-value: 0.014) while 100% of R/R patients with NPM1 mutated AML achieved CRc. 74% (N=17) of R/R patients in 1st salvage achieved CRc, with 53% proceeding to HSCT.
After a median follow up of 11 mo., median OS and EFS for the study cohort was not reached (NR) and 16 months, respectively. 1-year OS was 92% in ND, and 52% in R/R patients in salvage #1. Median DOR was 6 months in P1b and NR in P2A and P2B. OS was increased in patients achieving a MRD- CRc (OS: MRD- vs. MRD+: NR vs. 13 months, p-value: 0.038). 52% of patients (N=32) were successfully bridged to HSCT, including 5 patients receiving a second HSCT. 30 and 60-day post-HSCT mortality were both 0% and 1-year post-HSCT OS was 90% in ND, and 75% in R/R AML.
Median OS was NR in ND de novo and sAML, and 11 months in R/R AML. Inferior OS was observed in R/R patients with TS (6 vs 14 mo. p-value: 0.01) and active signaling (RAS, FLT3, PTPN11, KIT) (6 vs 16 mo. p-value: 0.018) mutations. Median OS was NR in patients with KMT2A rearrangements (N=7) or extra-medullary AML (N=4).
Conclusions
The addition of VEN to FLAG-IDA demonstrated robust efficacy across AML subgroups with an acceptable safety profile, an ORR of 84%, and 76% of subjects achieving a CRc (ND: 89%, R/R: 66%). FLAG-IDA-VEN resulted in deep responses as indicated by MRD- CRs in 83% of patients achieving a CR (ND: 96%, R/R: 70%). FLAG-IDA-VEN represents an effective regimen, particularly in adverse risk ND and R/R AML patients and as an effective bridge to HSCT.
Konopleva:Cellectis: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; Ablynx: Research Funding; Forty-Seven: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Agios: Research Funding; Kisoji: Consultancy; AstraZeneca: Research Funding; Calithera: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Ascentage: Research Funding; AbbVie: Consultancy, Research Funding; Sanofi: Research Funding; Amgen: Consultancy; Eli Lilly: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding. Kadia:Abbvie: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Astra Zeneca: Research Funding; Cellenkos: Research Funding; Pfizer: Honoraria, Research Funding; Astellas: Research Funding; Ascentage: Research Funding; Pulmotec: Research Funding; Amgen: Research Funding; Novartis: Honoraria; Genentech: Honoraria, Research Funding; Celgene: Research Funding; Incyte: Research Funding; JAZZ: Honoraria, Research Funding; Cyclacel: Research Funding. Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Short:Takeda Oncology: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria; Astellas: Research Funding. Sasaki:Novartis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Otsuka: Honoraria; Pfizer Japan: Consultancy. Borthakur:AstraZeneca: Research Funding; PTC Therapeutics: Consultancy; GSK: Research Funding; BMS: Research Funding; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding; BioTherix: Consultancy; Polaris: Research Funding; Xbiotech USA: Research Funding; Oncoceutics: Research Funding; Curio Science LLC: Consultancy; FTC Therapeutics: Consultancy; Argenx: Consultancy; PTC Therapeutics: Research Funding; BioLine Rx: Consultancy; Jannsen: Research Funding; BioLine Rx: Research Funding; Cyclacel: Research Funding. Issa:Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Research Funding; Celegene: Research Funding. Pemmaraju:Affymetrix: Other: Grant Support, Research Funding; Pacylex Pharmaceuticals: Consultancy; Stemline Therapeutics: Honoraria, Research Funding; LFB Biotechnologies: Honoraria; AbbVie: Honoraria, Research Funding; MustangBio: Honoraria; Daiichi Sankyo: Research Funding; Plexxikon: Research Funding; SagerStrong Foundation: Other: Grant Support; Incyte Corporation: Honoraria; Cellectis: Research Funding; Celgene: Honoraria; DAVA Oncology: Honoraria; Blueprint Medicines: Honoraria; Novartis: Honoraria, Research Funding; Roche Diagnostics: Honoraria; Samus Therapeutics: Research Funding. Jain:Pfizer: Research Funding; ADC Therapeutics: Research Funding; Incyte: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding. Yilmaz:Pfizer: Research Funding; Pint Pharma: Honoraria; Daicho Sankyo: Research Funding. Jabbour:Amgen: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding. Garcia-Manero:Celgene: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Merck: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; AbbVie: Honoraria, Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; H3 Biomedicine: Research Funding; Amphivena Therapeutics: Research Funding; Onconova: Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy. Ravandi:Orsenix: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Xencor: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding. Kantarjian:Aptitute Health: Honoraria; Janssen: Honoraria; Abbvie: Honoraria, Research Funding; BioAscend: Honoraria; Delta Fly: Honoraria; Amgen: Honoraria, Research Funding; Jazz: Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; BMS: Research Funding; Ascentage: Research Funding; Immunogen: Research Funding; Adaptive biotechnologies: Honoraria; Oxford Biomedical: Honoraria; Novartis: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Sanofi: Research Funding. Dinardo:Takeda: Honoraria; Daiichi Sankyo: Consultancy, Research Funding; ImmuneOnc: Honoraria; Novartis: Consultancy; Notable Labs: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Calithera: Research Funding; Agios: Consultancy, Research Funding; Celgene: Research Funding.
The BCL-2 inhibitor venetoclax will be combined with intensive chemotherapy (FLAG-IDA)
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract